Source: AFP
From the newsletter
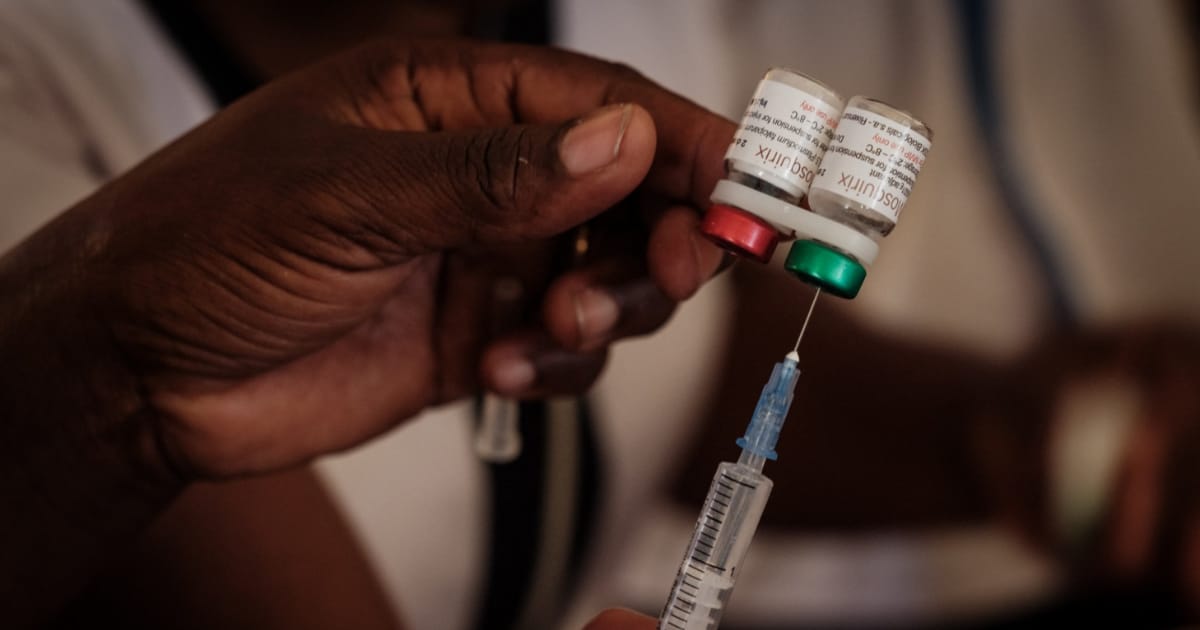
An American government demand that vaccines should no longer contain thimerosal, a preservative, could force a phase out of multi-dose vials which are cost friendly to produce for low and middle-income countries. Multi-dose vials require preservative. Phasing them out will drive up production costs and strain vaccine supplies for Africa.
Western vaccine standards ban certain preservatives, despite decades of research showing no harm.
Multi-dose vials are widely used in African immunisation programmes. They hold 2 to 20 doses to lower costs in production and simplify cold-chain logistics.
More details
The US request applies to both outstanding and future funding for Gavi, including $300 million previously approved by the Congress but not yet disbursed. The United States was historically responsible for about 13% of Gavi’s funding, making its conditions financially significant for vaccine procurement and planning in low- and middle-income countries.
Thimerosal is primarily used in multi-dose vaccine vials to prevent microbial contamination after opening. These formats are cheaper to manufacture, transport and store than single-dose vials. According to the World Health Organization, multi-dose vials are critical for routine immunisation and mass campaigns in low-resource settings due to reduced cold-chain volume and lower per-dose costs.
Gavi has said any decision to remove thimerosal-containing vaccines would require approval from its board and technical committees, guided by scientific consensus. The organisation confirmed it remains in discussions with the US government but has not committed to a phase-out plan, citing governance processes and the need to assess supply and programme implications.
Studies reviewed by the World Health Organization have found no evidence linking thimerosal, which contains ethyl mercury, to adverse health outcomes. The preservative has been largely phased out in high-income countries due to shifts toward single-dose formats, rather than safety bans, and remains permitted under international vaccine safety guidelines.
Any shift away from thimerosal would likely accelerate a move toward single-dose vaccine formats, which require higher manufacturing volumes and greater cold-chain capacity. One in four health facilities in Africa operate without electricity with several others operating on unreliable power. This means millions of children are already missing out on routine immunisation because of broken cold chains.
Our take
Western vaccine standards are being imposed on systems that lack Western infrastructure. Single-dose formats assume reliable electricity and dense cold chains which are not available in Africa.
In most parts of Africa, those assumptions do not hold and can lead to delivery risks with real coverage implications.